The law of activity change during radioactive decay. Basic law of radioactive decay
Necessary condition radioactive decay is that the mass of the original nucleus must exceed the sum of the masses of the decay products. Therefore, each radioactive decay occurs with the release of energy.
Radioactivity divided into natural and artificial. The first refers to radioactive nuclei that exist in natural conditions, the second - to the kernels obtained by nuclear reactions in laboratory conditions. Fundamentally, they do not differ from each other.
The main types of radioactivity include α-, β- and γ-decays. Before characterizing them in more detail, let us consider the law of the course of these processes in time common to all types of radioactivity.
Identical nuclei undergo decay at different times, which cannot be predicted in advance. Therefore, we can assume that the number of nuclei decaying in a short period of time dt, proportional to the number N available nuclei at that moment, and dt:
Integration of equation (3.4) gives:Relation (3.5) is called the basic law of radioactive decay. As you can see, the number N of yet undecayed nuclei decreases exponentially with time.
The intensity of radioactive decay is characterized by the number of nuclei decaying per unit time. It can be seen from (3.4) that this quantity | dN / dt | = λN. It's called activity. A. Thus activity:
 . .
|
It is measured in becquerels (Bq), 1 Bq = 1 decay / s; and also in curie (Ci), 1 Ci = 3.7∙10 10 Bq.
Activity per unit mass of a radioactive preparation is called specific activity.
Let us return to formula (3.5). Along with constant λ and activity A the process of radioactive decay is characterized by two more quantities: the half-life T 1/2 and average life time τ kernels.
Half life T 1/2- the time for which the initial number of radioactive nuclei on average will decrease by two:
 , ,
|
| . |
Average life time τ we define as follows. Number of cores δN(t) that experienced decay over a period of time ( t, t + dt), is determined by the right side of expression (3.4): δN(t) = λNdt. The lifetime of each of these nuclei is t. So the sum of the lifetimes of all N0 of the initially available nuclei is determined by integrating the expression tδN(t) in time from 0 to ∞. Dividing the sum of the lifetimes of all N0 cores per N0, we will find the average lifetime τ the kernel in question:
notice, that τ equals, as follows from (3.5), the time interval during which the initial number of nuclei decreases in e once.
Comparing (3.8) and (3.9.2), we see that the half-life T 1/2 and mean lifetime τ have the same order and are related by the relation:
 . .
|
Complex radioactive decay
Complex radioactive decay can occur in two cases:
physical meaning of these equations is that the number of nuclei 1 decreases due to their decay, and the number of nuclei 2 is replenished due to the decay of nuclei 1 and decreases due to its own decay. For example, at the initial time t= 0 available N01 cores 1 and N02 kernels 2. With such initial conditions, the solution of the system has the form:
If at the same time N02= 0, then
 . .
|
To evaluate the value N 2(t) you can use the graphical method (see Figure 3.2) for plotting curves e−λt and (1 − e−λt). In this case, due to the special properties of the function e−λt it is very convenient to plot the ordinates of the curve for the values t corresponding T, 2T, … etc. (see table 3.1). Relationship (3.13.3) and Figure 3.2 show that the amount of radioactive daughter increases with time and t >> T2 (λ 2 t>> 1) approaches its limit value:
and is called the age-old, or secular balance. The physical meaning of the secular equation is obvious.
| t | e−λt | 1 − e − λt |
| 0 | 1 | 0 |
| 1T | 1/2 = 0.5 | 0.5 |
| 2T | (1/2) 2 = 0.25 | 0.75 |
| 3T | (1/2) 3 = 0.125 | 0.875 |
| ... | ... | ... |
| 10T | (1/2) 10 ≈ 0.001 | ~0.999 |
 Figure 3.3. Complex radioactive decay. Figure 3.3. Complex radioactive decay.
|
Since, according to equation (3.4), λN is equal to the number of decays per unit time, then the relation λ 1 N 1 = λ 2 N 2 means that the number of decays of the daughter substance λ 2 N 2 is equal to the number of decays of the parent substance, i.e. the number of nuclei of the daughter substance formed in this case λ 1 N 1. The secular equation is widely used to determine the half-lives of long-lived radioactive substances. This equation can be used when comparing two mutually converting substances, of which the second has a much shorter half-life than the first ( T2 << T1) provided that this comparison is made at time t >> T2 (T2 << t << T1). An example of the successive decay of two radioactive substances is the transformation of radium Ra into radon Rn. It is known that 88 Ra 226, emitting with a half-life T1 >> 1600 yearsα-particles, turns into radioactive gas radon (88 Rn 222), which is itself radioactive and emits α-particles with a half-life T2 ≈ 3.8 days. In this example just T1 >> T2, so for times t << T1 the solution of equations (3.12) can be written in the form (3.13.3). |
For further simplification, it is necessary that the initial number of cores Rn be equal to zero ( N02= 0 at t= 0). This is achieved by a special setting of the experiment, in which the process of transformation of Ra into Rn is studied. In this experiment, the Ra preparation is placed in a glass flask with a tube connected to a pump. During the operation of the pump, the released gaseous Rn is immediately pumped out, and its concentration in the cone is zero. If at some point while the pump is running, the cone is isolated from the pump, then from that moment, which can be taken as t= 0, the number of nuclei Rn in the cone will begin to increase according to the law (3.13.3): N Ra and N Rn- accurate weighing, and λRn- by determining the half-life Rn, which has a value of 3.8, convenient for measurements days. So the fourth value λRa can be calculated. This calculation gives for the half-life of radium TRa ≈ 1600 years, which coincides with the results of the determination TRa by the method of absolute counting of emitted α-particles.
The radioactivity of Ra and Rn was chosen as a reference when comparing the activities of various radioactive substances. Per unit of radioactivity - 1 Key- accepted activity of 1 g of radium or an amount of radon that is in equilibrium with it. The latter can be easily found from the following reasoning.
It is known that 1 G radium undergoes ~3.7∙10 10 per second decays. Consequently.
Change in the number of radioactive nuclei over time. Rutherford and Soddy in 1911, summarizing the experimental results, showed that the atoms of some elements undergo successive transformations, forming radioactive families, where each member arises from the previous one and, in turn, forms the next one.
This can be conveniently illustrated by the example of the formation of radon from radium. If placed in a sealed ampoule, then an analysis of the gas after a few days will show that helium and radon appear in it. Helium is stable, and therefore it accumulates, while radon itself decays. Curve 1 in fig. 29 characterizes the decay law of radon in the absence of radium. At the same time, the ratio of the number of undecayed radon nuclei to their initial number is plotted on the y-axis. It can be seen that the content decreases exponentially. Curve 2 shows how the number of radioactive radon nuclei changes in the presence of radium.
Experiments carried out with radioactive substances showed that no external conditions (heating to high temperatures,
magnetic and electric fields, high pressures) cannot affect the nature and rate of decay.
Radioactivity is a property of the atomic nucleus and for a given type of nuclei in a certain energy state, the probability of radioactive decay per unit time is constant.

Rice. 29. Dependence of the number of active radon nuclei on time
Since the decay process is spontaneous (spontaneous), the change in the number of nuclei due to decay over a period of time is determined only by the number of radioactive nuclei at the moment and is proportional to the time interval
![]()
where is a constant characterizing the decay rate. Integrating (37) and assuming that we get
![]()
i.e., the number of nuclei decreases exponentially.
This law refers to statistical averages and is valid only for a sufficiently large number of particles. The value of X is called the radioactive decay constant, has a dimension and characterizes the probability of the decay of one atom in one second.
To characterize radioactive elements the concept of half-life is also introduced. It is understood as the time during which half of the available number of atoms decays. Substituting the condition into equation (38), we obtain
![]()
whence, taking logarithms, we find that
and half life
![]()
With the exponential law of radioactive decay, at any time there is a non-zero probability of finding nuclei that have not yet decayed. The lifetime of these nuclei exceeds
On the contrary, other nuclei that have decayed by this time have lived for different times, the shorter average lifetime for a given radioactive isotope is defined as

Denoting we get

Consequently, the average lifetime of a radioactive nucleus is equal to the reciprocal of the decay constant R. Over time, the initial number of nuclei decreases by a factor.
To process the experimental results, it is convenient to represent Eq. (38) in another form:
![]()
The value is called the activity of a given radioactive preparation, it determines the number of disintegrations per second. Activity is a characteristic of the entire decaying matter, and not of a single nucleus. The practical unit of activity is the curie. 1 curie is equal to the number of decayed nuclei contained in radium in 1 sec of decays/sec). Smaller units, millicuries and microcuries, are also used. In the practice of a physical experiment, sometimes another unit of activity is used - Rutherford disintegrations/sec.
Statistical nature of radioactive decay. Radioactive decay is a fundamentally statistical phenomenon. We cannot say exactly when a given nucleus will decay, but we can only indicate with what probability it decays over a given period of time.
Radioactive nuclei do not "age" in the course of their existence. The concept of age is generally inapplicable to them, but one can only talk about the average time of their life.
It follows from the statistical nature of the law of radioactive decay that it is strictly observed when it is large, and when it is small, fluctuations should be observed. The number of decaying nuclei per unit time must fluctuate around the average value, which is characterized by the above law. This is confirmed by experimental measurements of the number of -particles emitted radioactive substance per unit of time.

Rice. 30. Dependence of the logarithm of activity on time
Fluctuations obey Poisson's law. When making measurements with radioactive preparations, one must always take this into account and determine the statistical accuracy of the experimental results.
Determination of the decay constant X. When determining the decay constant X of a radioactive element, the experiment is reduced to registering the number of particles emitted from the drug per unit time, i.e., its activity is determined. Then a graph of the change in activity over time is plotted, usually on a semi-logarithmic scale. The form of dependences obtained in studies of a pure isotope, a mixture of isotopes, or a radioactive family turns out to be different.
Let's take a few cases as an example.
1. We study one radioactive element, the decay of which produces stable nuclei. Taking the logarithm of expression (41), we obtain
Therefore, in this case, the logarithm of activity is a linear function of time. The graph of this dependence has the form of a straight line, the slope of which (Fig. 30)
2. A radioactive family is being investigated, in which a whole chain of radioactive transformations occurs. The nuclei resulting from the decay, in turn, themselves turn out to be radioactive:
An example of such a chain is the decay:

Let us find a law describing in this case the change in the number of radioactive atoms in time. For simplicity, we single out only two elements: considering A as the initial one, and B as an intermediate one.
Then the change in the number of nuclei A and nuclei B will be determined from the system of equations
The number of nuclei A decreases due to their decay, and the number of nuclei B decreases due to the decay of nuclei B and increases due to the decay of nuclei A.
If at there are nuclei A, but there are no nuclei B, then the initial conditions will be written in the form
The solution of equations (43) has the form
and the total activity of the source, consisting of nuclei A and B:
Let us now consider the dependence of the logarithm of radioactivity on time for different ratios between and
1. The first element is short-lived, the second is long-lived, i.e. . In this case, the curve showing the change in the total activity of the source has the form shown in Fig. 31, a. At the beginning, the course of the curve is determined mainly by a rapid decrease in the number of active nuclei, the B nuclei also decay, but slowly, and therefore their decay does not greatly affect the slope of the curve in the section . In the future, there are few nuclei of type A in the mixture of isotopes, and the slope of the curve is determined by the decay constant. To determine the value, it is also necessary to take into account the influence of the decay of a long-lived element on the slope of the first part of the curve. To do this, the straight line is extrapolated to the region of short times, at several points the activity determined by element B is subtracted from the total activity according to the obtained values
they build a straight line for element A and find it by the angle (in this case, it is necessary to switch from logarithms to antilogarithms and vice versa).

Rice. 31. Dependence of the logarithm of the activity of a mixture of two radioactive substances on time: a - at at
2. The first element is long-lived, and the second is short-lived: The dependence in this case has the form shown in fig. 31b. At the beginning, the activity of the drug increases due to the accumulation of B nuclei. Then a radioactive equilibrium occurs, at which the ratio of the number of A nuclei to the number of B nuclei becomes constant. This type of equilibrium is called transitional. After some time, both substances begin to decrease at the rate of decay of the parent element.
3. The half-life of the first isotope is much longer than the second (it should be noted that the half-life of some isotopes is measured in millions of years). In this case, after a while, the so-called secular equilibrium is established, in which the number of nuclei of each isotope is proportional to the half-life of this isotope. Ratio
>> Law of radioactive decay. Half life
§ 101 LAW OF RADIOACTIVE DECAY. HALF LIFE
Radioactive decay obeys a statistical law. Rutherford, investigating the transformation of radioactive substances, established empirically that their activity decreases with time. This was discussed in the previous paragraph. Thus, the activity of radon decreases by 2 times after 1 min. The activity of elements such as uranium, thorium and radium also decreases with time, but much more slowly. For each radioactive substance, there is a certain time interval during which the activity decreases by 2 times. This interval is called the half-life. The half-life T is the time during which half of the initial number of radioactive atoms decays.
The decline in activity, i.e., the number of disintegrations per second, depending on time for one of the radioactive preparations is shown in Figure 13.8. The half-life of this substance is 5 days.
We now derive the mathematical form of the law of radioactive decay. Let the number of radioactive atoms at the initial time (t= 0) be N 0 . Then after the half-life period, this number will be equal to
After another similar time interval, this number will become equal to:

The radioactive decay of the nuclei of the same element occurs gradually and with different speed for different radioactive elements. It is impossible to specify in advance the moment of the decay of the nucleus, but it is possible to establish the probability of the decay of one nucleus per unit of time. The probability of decay is characterized by the coefficient "λ" - the decay constant, which depends only on the nature of the element.
Law of radioactive decay.(Slide 32)
It has been experimentally established that:
For equal intervals of time, the same proportion of the available (ie, not yet decayed by the beginning of this interval) nuclei of a given element decays.
Differential form of the law of radioactive decay.(slide 33)
Sets the dependence of the number of undecayed atoms in this moment time from the initial number of atoms at the zero moment of the reference point, as well as from the decay time "t" and the decay constant "λ".
N t - available number of cores.
dN is the decrease in the available number of atoms;
dt is the decay time.
dN ~ N t dt Þ dN = –λ N t dt
"λ" - coefficient of proportionality, decay constant, characterizes the share of available, not yet decayed nuclei;
"–" - says that over time, the number of decaying atoms decreases.
Consequence #1:(slide 34)
λ = –dN/N t dt - relative rate of radioactive decay for given substance is a constant value.
Consequence #2:
dN/N t = – λ · Nt - the absolute rate of radioactive decay is proportional to the number of non-decayed nuclei by the time dt. It is not "const" because decrease over time.
4. Integral form of the law of radioactive decay.(slide 35)
Sets the dependence of the number of remaining atoms at a given time (N t) on their initial number (N o), time (t) and decay constant "λ". The integral form is obtained from the differential:
1. Separate the variables:

2. We integrate both parts of the equality:

3. Find integrals  Þ
Þ  -common decision
-common decision
4. Find a particular solution:
If a t = t 0 = 0 Þ N t = N 0 , we substitute these conditions into the general solution
(start(original number
decay) of atoms)
 Þ
Þ
 In this way:
In this way:
 integral form of the law p/act. decay
integral form of the law p/act. decay
N t - the number of atoms that have not decayed by the time t ;
N0 - initial number of atoms at t = 0 ;
λ - decay constant;
t - decay time
Conclusion: The available number of undecayed atoms is ~ the initial number and decreases over time according to an exponential law. (slide 37)
Nt= N 0 2 λ 1 λ 2 >λ 1 Nt = N 0 e λ t
5. Half-life and its relation to the decay constant. ( slide 38.39)
The half-life (T) is the time during which half of the original number of radioactive nuclei decays.
It characterizes the decay rate of various elements.
Basic conditions for the definition of "T":
1. t \u003d T - half-life.
2.  - half of the original number of cores for "T".
- half of the original number of cores for "T".
The connection formula can be obtained if these conditions are substituted into the integral form of the law of radioactive decay

1. 
2. Reduce "N 0". Þ 
3. 
4. Potentiate.
 Þ
Þ 
5. 
The half-life of isotopes varies widely: (slide 40)
238 U ® T = 4.51 10 9 years
60 Co ® T = 5.3 years
24 Na ® T = 15.06 hours
8 Li ® T = 0.84 s
6. Activity. Its types, units of measurement and quantification. activity formula.(slide 41)
In practice, it is of paramount importance total number decays attributable to the source radioactive radiation per unit time => quantitatively determine the measure of decay activity radioactive substance.
The activity (A) depends on the relative decay rate "λ" and on the available number of nuclei (ie, on the mass of the isotope).
"A" - characterizes the absolute decay rate of the isotope.
3 options for writing the activity formula: (slide 42.43)
I. From the law of radioactive decay to differential form follows:
 Þ
Þ 

 activity (the absolute rate of radioactive decay).
activity (the absolute rate of radioactive decay).
 activity
activity
II. From the law of radioactive decay in integral form it follows:

1.  (multiply both sides of the equality by "λ").
(multiply both sides of the equality by "λ").
Þ 
2.  ;
;  (initial activity at t = 0)
(initial activity at t = 0)
3.  the decline in activity follows an exponential law
the decline in activity follows an exponential law
III. When using the formula for the relationship of the decay constant "λ" with the half-life "T" follows:

1.  (we multiply both sides of the equality by " N t
" to get the activity). Þ
(we multiply both sides of the equality by " N t
" to get the activity). Þ  and get the formula for the activity
and get the formula for the activity
2. 
Activity units:(slide 44)
BUT. System units.
A = dN/dt
1[disp/s] = 1[Bq] – becquerel
1Mdisp/s = 10 6 dispersal/s = 1 [Rd] - rutherford
B. Non-system units of measure.
[Ki] - curie(corresponds to the activity of 1 g of radium).
1[Ci] = 3.7 10 10 [disp/s]- in 1 g of radium, 3.7 10 10 radioactive nuclei decay in 1 s.
Activities:(slide 45)
1. Specific is the activity per unit mass of a substance.
And ud. = dA/dm [Bq/kg].
It is used to characterize powdered and gaseous substances.
2. Volumetric is the activity per unit volume of a substance or medium.
A about \u003d dA / dV [Bq / m 3]
It is used to characterize liquid substances.
In practice, the decrease in activity is measured using special radiometric instruments. For example, knowing the activity of the drug and the product formed during the decay of 1 nucleus, it is possible to calculate how many particles of each type the drug emits in 1 second.
If during the fission of the nucleus neutrons "n" are formed, then a stream of neutrons "N" is emitted in 1 s. N = n A.
©2015-2019 site
All rights belong to their authors. This site does not claim authorship, but provides free use.
Page creation date: 2016-08-08
§ 15-f. Law of radioactive decay
The advent of "manual" scintillation counters, and especially Geiger-Muller counters, which helped to automate particle counting (see § 15th), led physicists to an important conclusion. Any radioactive isotope is characterized by a spontaneous weakening of radioactivity, expressed in a decrease in the number of decaying nuclei per unit time.
Plotting the activity of various radioactive isotopes led scientists to the same dependence, expressed exponential function(see graph). The time of observation is plotted along the horizontal axis, and the number of undecayed nuclei is plotted along the vertical axis. The curvature of the lines could be different, but the function itself, which expressed the dependencies described by the graphs, remained the same:
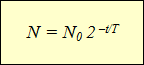
This formula expresses radioactive decay law: the number of nuclei that have not decayed over time is defined as the product of the initial number of nuclei by 2 to a power equal to the ratio of the observation time to the half-life, taken with a negative sign.
As it turned out in the course of experiments, various radioactive substances can be characterized by different half-life- the time during which the number of still undecayed nuclei is halved(see table).
Half-lives of some isotopes of some chemical elements. Values are given for both natural and artificial isotopes.
| Iodine-129 | 15 Ma | Carbon-14 | 5.7 thousand years | |
| Iodine-131 | 8 days | Uranium-235 | 0.7 Ga | |
| Iodine-135 | 7 o'clock | Uranium-238 | 4.5 billion years |
Half-life is generally accepted physical quantity characterizing the rate of radioactive decay. Numerous experiments show that even with a very long observation of a radioactive substance, its half-life is constant, that is, it does not depend on the number of already decayed atoms. Therefore, the law of radioactive decay has found application in the method of determining the age of archaeological and geological finds.
Method of radiocarbon analysis. Carbon is very abundant on Earth chemical element, which includes stable isotopes carbon-12, carbon-13 and a radioactive isotope carbon-14, the half-life of which is 5.7 thousand years (see table). Living organisms, consuming food, accumulate all three isotopes in their tissues. After the end of the life of the organism, the supply of carbon stops, and over time, its content decreases naturally, due to radioactive decay. Since only carbon-14 decays, the ratio of carbon isotopes in the fossil remains of living organisms changes over the course of centuries and millennia. By measuring this "carbon proportion", one can judge the age of the archaeological find.
The method of radiocarbon analysis is also applicable to geological rocks, as well as to fossil household items, but on condition that the ratio of isotopes in the sample has not been disturbed during its existence, for example, by a fire or the action of a strong source of radiation. Failure to take into account such reasons immediately after the discovery of this method led to errors for several centuries and millennia. Today, "centennial calibration scales" are used for the carbon-14 isotope, based on its distribution in long-lived trees (for example, in the American thousand-year-old sequoia). Their age can be calculated quite accurately - according to the annual rings of wood.
The limit of application of the method of radiocarbon analysis at the beginning of the 21st century was 60,000 years. To measure the age of older specimens, for example rocks or meteorites, use a similar method, but instead of carbon, observe isotopes of uranium or other elements, depending on the origin of the sample being studied.
Javascript is disabled in your browser.ActiveX controls must be enabled in order to make calculations!